At The FDA Group, we recognize that life science teams often collaborate with multiple vendors to complete their projects, which can be expensive, time-consuming, and complicated to manage.
We believe that life science teams deserve more than mere transactional vendor relationships; instead, they should have genuine partnerships that emphasize open communication, trust, and collaboration to effectively address all their needs.
Introducing our Functional Service Program
That's why we've created a Functional Service Program (FSP) — a way to consolidate some or even all your externally-sourced projects through a single, trusted partner with substantial discounts. Our FSP model simplifies vendor management, eradicates inefficiency, and minimizes budget overruns, all while delivering high-quality services from the industry's top talent.
Simply tell us which projects you'd like to outsource to us for a significant discount, and we'll develop a plan to resource and execute those projects with progressively lower rates. The more you outsource to us, the more you save. You'll have a single, dependable resourcing partner to plan, coordinate, and execute projects and programs at a fraction of the cost and complexity you currently manage.
With a single external support partner, you can more efficiently move and deploy the same resources across multiple functional areas, avoiding costs and delays due to additional onboarding and training.
Our FSP model caters to the later stages of the product lifecycle, where bundled service engagement offerings are scarce. Like a traditional clinical FSP, this outsourcing model allows you to streamline your external work and benefit from a single point of accountability and integrated service offering.
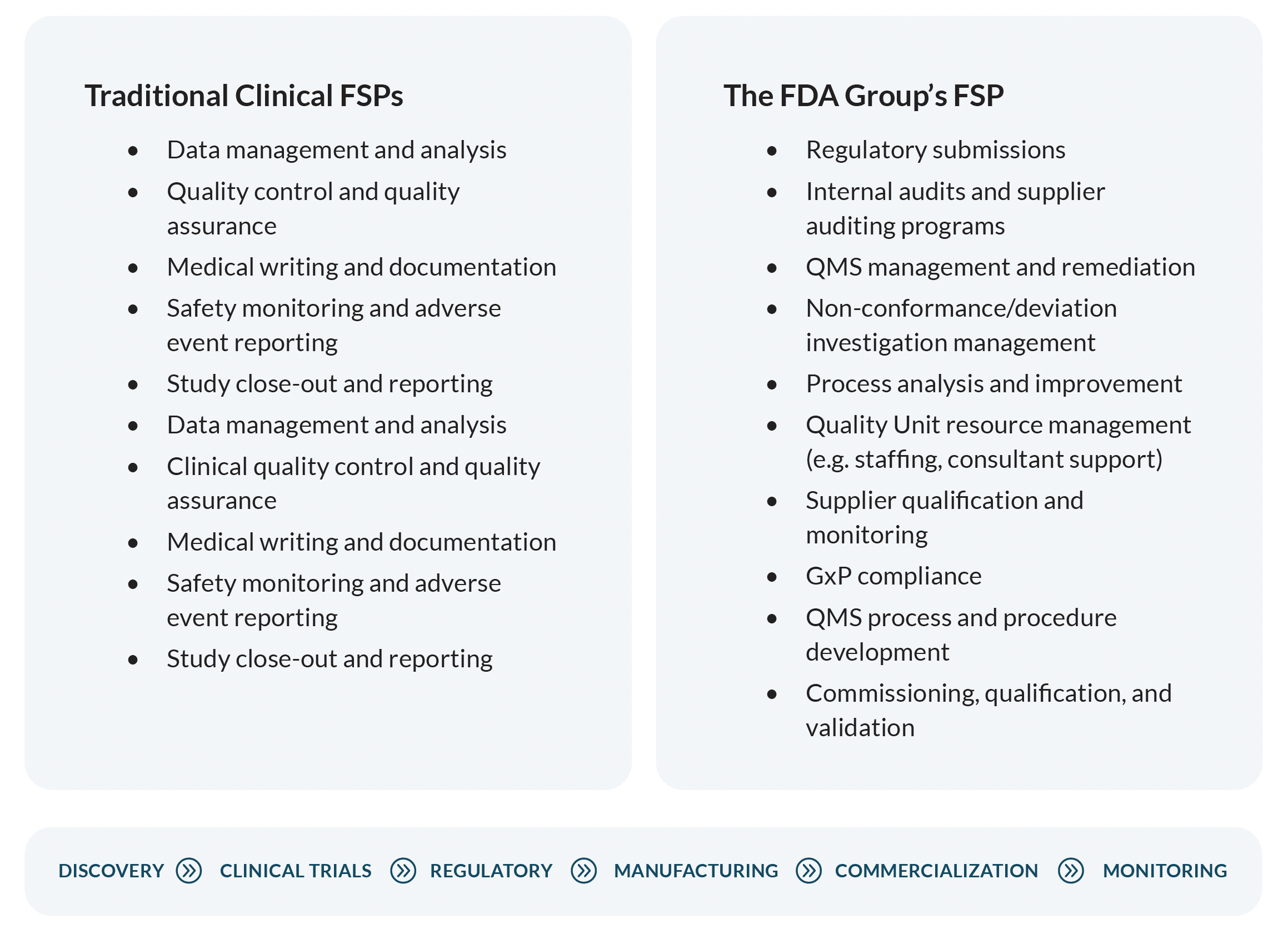
With discounts that increase with the size and duration of the service package, you'll cut costs and reduce management workload—all while receiving dependable service backed by a Total Quality Guarantee.
Functional Service Items
The tables below provide a selection of just some of the services you can bundle into a single, discounted service package over a given period. Contact us to express your interest and schedule a call to discuss service packaging, discounting, and our full range of services.
Quality Assurance
| Mock Pre-Approval Inspections (PAI) and mock FDA audits | GMP, GCP, and GLP audits |
| Mock Notified Body Inspections | Mock recalls |
| Vendor/supplier audits | MDSAP audits |
| Site investigator and clinical audits | Quality system and SOP gap analysis |
| Quality resource/workforce management (temp and permanent) | QMS development and remediation |
| Non-conformance/deviation investigation management support | Deviation backlog reduction |
| Process analysis and improvement | Investigation, action plan, and effectiveness checks |
| Risk assessments and risk mitigation strategies | Document control and change management |
| Regulatory compliance support (ISO, FDA regulations) | Complaint and adverse event handling |
| CAPA management and follow-up |
Regulatory Affairs
| Preparation and submission of NDA, BLA, PMA to FDA, or ANDA | Regulatory affairs resource/workforce management (temp and permanent) |
| Preparation of regulatory submissions for investigational devices and clinical trial applications | Preparation and submission of annual reports and amendments to regulatory agencies |
| Development and implementation of regulatory strategies for product development and commercialization | Preparation and submission of regulatory reports and documents, such as adverse event reports and annual reports |
| Monitoring of regulatory developments and guidance and providing advice to clients on regulatory compliance and issues | Preparation of regulatory submissions for orphan drug designation |
| Preparation of labeling and promotional material in accordance with FDA regulations and guidance | Document control and change management |
| EU MDR and IVDR compliance |
Commissioning, Qualification and Validation
| Commissioning, qualification and validation resource/workforce management (temp and permanent) | DQ, IQ, OQ, PQ |
| Equipment Validation | Software Validation |
| Process Validation |
Get in touch to start the conversation
If you're interested in learning more about this bundled engagement option, head to our FSP engagement model page and contact us to schedule a brief discussion to explore it further. We'll answer your questions, identify potential bundled service opportunities, and plan the next steps.
Want a more in-depth overview of the problem our FSP solves, how it operates, and the types of services you can bundle through it? Read our overview guide below.
 FREE GUIDE
FREE GUIDE
The FDA Group's Functional Service Program
Consolidate and bunder your externally sourced projects with The FDA Group for significantly discounted rates.

