The FDA Group's Insider Newsletter costs $29.99/month.
The FDA Group’s Insider Newsletter is a premium newsletter that delivers regulatory and compliance news, as well as highly-actionable analysis from former FDA staff and experienced life science consultants right to your inbox.
Subscribers keep up on news and other happenings at the FDA seen through the lens of those who’ve worked within the FDA and the industries it oversees. New issues will arrive each month.
Subscribe and get 50% off for 12 months » Sample the newsletter »
Each month, The FDA Group’s Insider Newsletter delivers three regulatory and compliance news analyzed by former FDA staff and experienced life science consultants working in the FDA-regulated industries.
Each newsletter contains a subscriber-only video of the expert contributor’s analysis with a transcript of the video and links to relevant articles and resources on the web. Subscribers get actionable insights they won't find elsewhere.

There are a handful of free and paid regulatory intelligence services that report on new regulations, FDA guidances, enforcement actions, etc.
Many of these services do a decent job of putting need-to-know industry news in front of you, but few—if any—actually provide the expert analysis and perspectives that help you understand what you need to do in light of, say, new a guidance being issued. Even fewer go so far to break down warning letters and pick out the key lessons from them.
It’s up to you to digest the information, determine the impact on your organization, and plan accordingly. That takes time and attention that we suspect you could better invest elsewhere.
Once a month, we gather regulatory and compliance happenings and put them to a former FDA official or experienced consultant with the appropriate background to analyze that news and distill it into actionable takeaways for RA, QA, and Clinical teams.
They record their analysis, and we send it to you. It's that simple.
We hope it will save you time hunting for that news and understanding what you need to do as a result of it—all through the lens of someone with deep industry or agency experience. We cover new regulatory initiatives, dig into new guidances, pull out key themes of warning letters, and more.
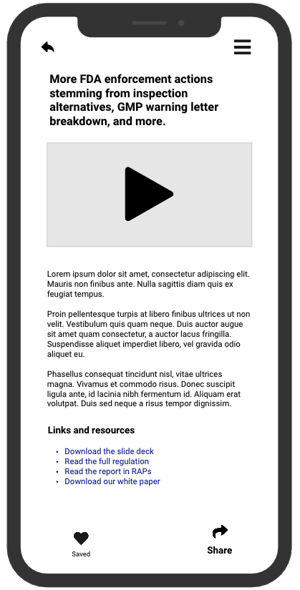
Watch our "pilot" edition and get a sense of what to expect each month. We've gathered a team of former FDA professionals and industry consultants as our expert contributors.
Subscribers will get exclusive access to videos like this every month—covering topics across the pharma, device, and biologics industries.
Our pilot features contributor Larry Stevens, RAC, a life science consultant who has held almost every field position within FDA during his 21-year career with the agency.
The FDA Group's Insider Newsletter costs $29.99/month.
Each newsletter will contain:
The newsletter will be hosted and delivered via Substack, a platform designed specifically for independent newsletter publishers.
Payments will be handled securely through Substack, which uses Stripe as its checkout platform.
At this time, Substack accepts most major credit cards such as Visa, MasterCard, Discover, and American Express.
Use this link to subscribe: thefdagroup.substack.com
Yes, of course. Substack makes it very easy to cancel any paid subscription. Once you cancel your subscription, it will terminate at the end of the billing period and you will not be charged again.
We plan to cover news and topics relevant to RA/QA/Clinical professionals working in FDA-regulated industries. That includes new regulatory initiatives from FDA and other regulatory bodies, guidance document analyses, enforcement action breakdowns, and more. We'll be focusing on the pharmaceutical, medical device, and biologic industries in particular.
The Insider Newsletter will be unique in a few ways:
Regulatory affairs, quality assurance, and clinical operations teams need to stay on top of a fast-moving regulatory environment. Hunting for news and scouring databases steals valuable time that’s better spent elsewhere. The Insider Newsletter gathers important news so you don’t have to. Each month, we aggregate the latest relevant regulatory happenings and deliver expert analysis on key topics. Quickly scan regulatory updates from the FDA and stay for insights that help you take action on them.
It’s easy to get overwhelmed by the constant stream of regulatory information within the FDA-regulated industries. Keeping track of it all is hard enough. Understanding and responding to it thoughtfully is even harder. Our former FDA contributors analyze regulatory developments so you can focus on what it means for your organization and take efficient, effective action.
Proprietary talent selection of former FDA and industry professionals amplified by a corporate culture of responsiveness and execution.
US Toll-Free: 1-833-FDA-GROUP
International: +001 508 926 8330
