On October 31, 2017, The U.S. Food and Drug Administration (FDA) announced it will take the "unprecedented step" of recognizing drug manufacturing facility inspections around the world conducted by eight European agencies, in an effort to reduce duplicative oversight through greater collaboration with the EU member states.
FDA has agreed to rely on GMP inspection data gathered by drug regulators in Austria, Croatia, France, Italy, Malta, Spain, Sweden and the United Kingdom.
This new international recognition by FDA comes in response to similar steps taken by the European Commission, which in June of 2017 confirmed that FDA is capable of conducting GMP inspections equivalent to those conducted in the EU.
In its press release, FDA Commissioner Scott Gottlieb offered his thoughts behind the new initiative:
"At a time in which medical product manufacturing is truly a global enterprise, there is much to be gained by partnering with regulatory counterparts to reduce duplicative efforts and maximize global resources while realizing the greatest bang for our collective inspectional buck."
What's behind the move
The European Medicines Agency estimates that 85% of medicines sold in the EU have at least one step of the manufacturing process handled outside of the member states. Tighter cooperation between U.S. and EU regulators is a clear signal that regulating bodies are taking steps to structure their relationships to reflect the reality that many pharmaceutical products are manufactured — at least in part — outside of the U.S. and Europe.
Given that both regulatory authorities routinely inspect manufacturing facilities and suppliers to ensure quality standards are maintained, FDA can rely on information gathered by inspectors from any one of those eight agencies to assess whether sites are compliant.
Tighter oversight and control over foreign compliance
Over the past few years, FDA has boosted its oversight of foreign drug manufacturers, issuing numerous warning letters to facilities operating below established and expected GMP standards.
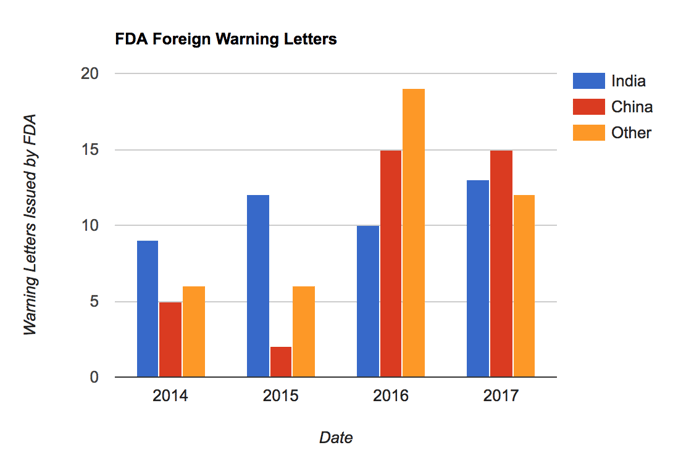
This closer coordination comes almost two decades after a 1998 agreement which first presented possibility of mutually recognizing GMP inspections between the U.S. and the EU. The annex governing such collaboration was never fully implemented until now.
FDA plans to complete its capability assessment of all remaining EU countries by July 2019. This lays out the prospect for recognition of additional regulators beyond the eight identified on Oct. 31.