With the clock ticking toward 2020, MDR transition should be a high priority for device companies currently marketing products—or planning to market products—in the EU. Due to significant changes in the forthcoming Regulation versus the MDD, this transition process should begin with a comprehensive gap assessment led by experienced compliance experts who know exactly what to look for.
Since the MDR does not grandfather legacy products and presents limited allowance on the short-term continuation of supplies to the EU market following the formal transition, portfolio revision and a global impact assessment will also be needed for many organizations.
Once these initial product decisions and process steps are taken, transition largely becomes a matter of training staff and implementing the changes needed. We’ve broken this down into seven essential steps along with insight into how an experienced quality and compliance specialist can play vital role in your transition.
In this article, an excerpt from our recent white paper, The Complete Guide to EU-MDR Transition, we explore the first of these seven steps.
Grab our free white paper and learn all seven steps to an effective EU-MDR transition program ⤵
The Complete Guide to EU-MDR Transition
Step 1: Plan and Scope Your Transition Program
Understanding the breadth required of your MDR transition program from a purely practical perspective is an essential first step. This will inform the budget needed to build out project teams and layout tasks in front of them.
While scoping and planning a project like this may not seem particularly new or challenging to those leading your transition, be cautious to avoid presenting the false impression that MDR doesn’t depart significantly from MDD in key areas. Downplaying the differences sets a dangerous tone and can degrade the sense of urgency needed to execute your plan effectively.
While it’s true that like the MDD, the MDR requires companies maintain a full quality system and technical files for each product marketed in Europe with pre-market reviews carried out by the same Notified Bodies, these similarities shouldn’t cast a shadow on the essential changes just mentioned, as well as one of the most important changes at the foundation of the regulation:
The legal bases of MDR will switch to a central EU regulation, replacing directives instructing individual countries what to include in their laws.
The stated goal of this change is to help Notified Bodies and boost supply chain control, however, manufacturers should expect regulators to demand much more precise clinical data in support of the safety and performance of devices within their intended use.
For those leading the transition team, make sure to include the following three action items in this critical first phase:
- Make sure your entire project team reads the “whereas section” before article 1. This explains the specific goals of the law in clear and practical terms and should be used as a starting point for planning next actions.
- Make sure your entire project team studies the 35 pages of instructions addressed to the Notified Bodies. This will help everyone understand what Notified Bodies will be looking for. These instructions can be backwards-engineered to serve as a checklist when preparing for assessment.
- Use the quick-guide below to help you meet the stricter requirements for quality and clinical data. The MDR presents major changes to technical product validation that should be closely studied. To help device companies take steps toward meeting stricter requirements for this data, regulators have updated the guidance for current legislation to align more closely with MDR expectations. The main components of this change are summarized below.
Guidance MEDDEV 2.7.1 rev
• A Clinical Evaluation Report now applies for all classes of devices
• Benefit risk in intended use/target group
• Usability requirements must now be included
• Clinical data must now be integrated in lifecycle management
• Clinical evaluations must now be conducted before and after clinical studies
• Regulators will expect larger patient numbers in studies
• Expect a stronger focus on the analysis and appraisal of data
• Evaluators will be subject to qualification
• Updates must be made annually, every two years and five years
Along with enhancing clinical safety and performance, the MDR also makes key changes to Notified Bodies in order to focus their attention (and that of manufacturers) on compliance. Following the removal of 30 Notified Bodies and a significant reduction in scope of those remaining to focus on core areas of importance, device companies should prepare themselves accordingly.
Under MDR, Notified Bodies will have greater assessment powers and stronger systems for coordinating with authorities, who in turn, will have stronger supervision over these assessors. These changes pose important questions for device companies, chiefly: How do these changes practically impact my system?
A look at the broader MDR timeline shows that systemic change will be an almost decade-long process, broken into increments, with many of the more system-wide changes occurring during the soft transition phase from 2020 through 2025.
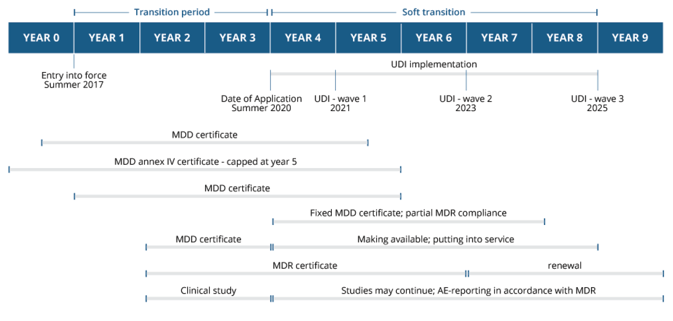
This post is an excerpt from our recent white paper, The Complete Guide to EU-MDR Transition.

Grab our free white paper and learn all seven steps of an effective EU-MDR transition program, along with key changes and areas of focus.


